Introduction: Endometriosis is characterized by the presence of functional endometrial tissue consisting of glands and/ or
stroma located outside the uterus [1], although implanted ectopically, this tissue presents histopathological and physiological
responses that are similar to the responses of the endometrium [2].
Clinical Features: Endometriosis usually becomes apparent in
the reproductive years when the lesions are stimulated by
ovarian hormones. Forty percent of the patient’s present symptoms in a
cyclic manner, which are usually related with menses Pelvic
pain, infertility and dyspareunia are the characteristic symptoms of the
disease, but the clinical presentation is often non-specific
[1].
Diagnosis and Investigations: A precise diagnosis about the
presence, location and extent of rectosigmoid endometriosis is
required during the preoperative workup because this information is
necessary in the discussion with both the colorectal surgeon
and the patient. Furthermore, almost all patients with intestinal
endometriosis have lesions in multiple pelvic locations and it is
difficult to know what symptoms are caused by the intestinal disease
versus the pelvic disease.
Treatment: Treatment must be individualized, taking the
clinical problem in its entirety into account, including the impact of
the disease and the effect of its treatment on quality of life. Pain
symptoms may persist despite seemingly adequate medical and/
or surgical treatment of the disease. In such circumstances, a
multi-disciplinary approach involving a pain clinic and counselling
should be considered early in the treatment plan.
Endometriosis is characterized by the presence of functional
endometrial tissue consisting of glands and/ or stroma located
outside the uterus [1], although implanted ectopically, this tissue
presents histopathological and physiological responses that are
similar to the responses of the endometrium [2].
The reported prevalence of endometriosis is 1%-20% in
asymptomatic women, 10%-25% in infertile patients and 60%-
70% in women with chronic pelvic pain [1]. Endometriosis is a
common benign disease among women of reproductive age and
affects the intestinal tract in 15%-37% of all patients with pelvic
endometriosis [3]. Multiple births and extended intervals of
lactation decrease the risk of being diagnosed with endometriosis,
whereas nulliparity, early menarche, frequent menses, and
prolonged menses increase the risk [4]. Endometriosis also
appears to be associated with a taller, thinner body habitus and
lower body mass index [5]. The prevalence appears to be lower in
blacks and Asians than in Caucasians [6]. Growth and maintenance
of endometriotic implants are dependent upon the presence of
ovarian steroids. As a result, endometriosis occurs during the active
reproductive period: women aged 25 to 35 years [6]. Other factors
that appear to play important roles in determining if a woman will
develop the clinical condition include [7]:
a) Reproductive lifestyle, especially a delay in childbearing
b) Poorly understood immunological factors
c) Some environmental factors, probably including exposure
to a range of environmental toxins
d) Reproductive tract occlusion, such as an imperforate
hymen.
Endometriosis is a common disease of unknown etiology. Many
theories have been proposed to explain this condition: retrograde
menstruation theory, metaplastic, transformation, the migration of
cells through the lymphatic system or via hematogenous spread,
Iatrogenic during CS. However, other factors, immunological,
genetic and familial, could be involved in the pathogenesis of this
disease [1].
Sampson’s Theory of Retrograde Menstruation
The implantation theory proposes that endometrial tissue
passes through the fallopian tubes during menstruation, then
attaches and proliferates at ectopic sites in the peritoneal cavity.
Recent studies using laparoscopy have demonstrated that
retrograde menstruation is a nearly universal phenomenon in
women with patent fallopian tubes. Classic studies performed in
the 1950s demonstrated viability of sloughed endometrial cells
and the capacity to implant at ectopic sites. Patients with mullerian
anomalies and obstructed menstrual flow through the vagina may
have an increased risk of endometriosis. The anatomic distribution
of endometriosis also provides evidence for Sampson’s theory [8].
Coelomic Metaplasia Theory
The theory of coelomic metaplasia proposes that endometriosis
may develop from metaplasia of cells lining the pelvic peritoneum.
Iwanoff and Meyer are recognized as originators of this theory. A
prerequisite of the coelomic metaplasia theory is that mesothelial
cells lining the ovary and pelvic peritoneum contain cells capable
of differentiating into endometrium. An attractive component
of the coelomic metaplasia theory is that it can account for the
occurrence of endometriosis anywhere mesothelium is found.
This includes reports of endometriosis occurring in the pleural
cavity. Pleural endometriosis could result from local metaplasia
of pleural mesothelium. On the other hand, it could also result
from transdiaphragmatic passage of peritoneal fragments of
endometrium as well as vascular metastasis of endometrium.
Coelomic metaplasia is thought to account for the rare occurrences of
endometriosis reported in males. In these reports of endometriosis,
the men were all undergoing estrogen therapy. Although coelomic
metaplasia was a possibility, estrogen stimulation of mullerian rests
could not be excluded. Likewise, the occurrence of endometrial
carcinoma in males is thought to possibly arise from mullerian
remnants. Still, further support for the coelomic metaplasia theory
may be found in the study of benign and malignant epithelial
ovarian tumors. Both are considered to be derivatives of germinal
epithelium. The presence of ovarian surface endometriosis could
be accounted for by this type of transformation [8].
Induction Theory
The induction theory is an extension of the coelomic metaplasia
theory. This theory proposes that menstrual endometrium produces
substances that induce peritoneal tissues to form endometriotic
lesions [8].
Embryonic Rests Theory
Von Recklinghausen and Russell are credited with the
theory that endometriosis results from embryonic cell rests.
These embryonic rests, when stimulated, could differentiate
into functioning endometrium. As described above, rare cases
of endometriosis have been reported in men. Transformation of
embryonic rests is a plausible explanation for this phenomenon [8].
Lymphatic and Vascular Metastasis Theories
The lymphatic metastasis theory of endometriosis is often
referred to as Hal ban’s theory. He reported that endometriosis
could arise in the retroperitoneum and in sites not directly opposed
to peritoneum. Sampson had also suggested that endometriosis
could result from lymphatic and hematogenous dissemination
of endometrial cells. An extensive communication of lymphatics
has been demonstrated between the uterus, ovaries, tubes, pelvic
and vaginal lymph nodes, kidney, and umbilicus. Metastasis
of endometrial cells via the lymphatic system to these areas is
therefore anatomically possible. These findings are consistent
with a literature review showing a 6.7% incidence of lymph node
endometriosis in 178 autopsy cases. Lymphatic and vascular
metastasis of endometrium has been offered as an explanation for
rare cases of endometriosis occurring in locations remote from the
peritoneal cavity. In addition to pleural tissue, endometriosis has
been reported in pulmonary parenchyma. Vascular or lymphatic
metastasis may also explain cases of endometriosis that have been
reported in bone, biceps muscle, peripheral nerves, and the brain
[8].
Composite Theory
Javert proposed a composite theory of the histogenesis of
endometriosis which combines the implantation, vascular/
lymphatic metastasis, as well as a theory of direct extension of
endometrial tissue through the myometrium. Along similar lines,
Nisolle and Donnez have recently argued that the histogenesis
of endometriosis depends on the location and ‘type’ of the
endometriotic implant. For example, peritoneal endometriosis can
be explained by the implantation theory. Ovarian endometriomas
could be the result of coelomic metaplasia of invaginated ovarian
epithelial inclusions. Rectovaginal endometriosis, which often
resembles adenomyosis, could result from metaplasia of Mullerian
remnants located in the rectovaginal septum. These composite
theories are attractive in that they recognize a multifaceted
mechanism of histogenesis. It seems logical that a disease with such
variable manifestations may originate via several mechanisms [8].
Altered Immunity
Alterations in immunologic response to retrograde
menstruation have been implicated in the genesis and maintenance
of the endometriotic lesion. This defective immunosurveillance may
lead to decreased clearance of menstrual debris from the peritoneal
cavity and may allow for attachment of ectopic endometrium to
peritoneal surfaces. An abnormal immune response could also
promote the persistence and growth of ectopic endometrial tissue
[8].
The “Neurologic Hypothesis”
It is a new concept in the pathogenesis of endometriosis:
There is a close histological relationship between endometriotic
lesions of the large bowel and the nerves of the large bowel wall.
Endometriotic lesions seem to infiltrate the large bowel wall
preferentially along the nerves, even at distance from the palpated
lesion, while the mucosa is rarely and only focally involved [9].
Sites
Endometriosis can be divided into intra- and extraperitoneal
sites. In decreasing order of frequency, the intra-peritoneal
locations are ovaries (30%), uterosacral and large ligaments
(18%-24%), fallopian tubes (20%), pelvic peritoneum, pouch
of Douglas, and gastrointestinal (GI) tract. Extra-peritoneal
locations include cervical portio (0.5%), vagina and rectovaginal
septum, round ligament and inguinal hernia sac (0.3%-0.6%),
navel (1%), abdominal scars after gynaecological surgery (1.5%)
and caesarian section (0.5%). Endometriosis rarely affects extraabdominal
organs such as the lungs, urinary system, skin and the
central nervous system [1]. Endometriosis affects the intestinal
tract in 15% to 37% of patients with pelvic endometriosis [10],
involvement have been reported from the small bowel to the anal
canal, but more frequently the disease involves the rectum and the
sigmoid colon (74%), followed by the rectovaginal septumn (12%),
cecum (2%), and appendix (3%) . When the ileum is involved, the
most common tract is the distal part. A full-thickness involvement
of the colonic wall is infrequent since the mucosa is usually spared.
One of the classic locations is the anterior rectal wall in the region
of the pouch of Douglas. This can be single nodule or can simulate
a cancer. Because of the invasive appearance, the disease can be
mistaken for cancer [11].
Gross and Microscopic Pathology of Bowel Endometriosis
The appearance and size of the implants are quite variable.
Areas of endometriosis appear as raised flame-like patches, whitish
opacifications, yellow-brown discoloration, translucent blebs, or
reddish or reddish-blue irregularly shaped islands. The peritoneal
surface may be scarred or puckered.
The Microscopic Appearance
Of endometriotic tissue is similar to that of endometrium in the
uterine cavity; the two major components of both are endometrial
glands and stroma. Unlike endometrium, however, endometriotic
implants often contain fibrous tissue, blood, and cysts (Figure 1(a)
& 1(b)).
Figure 1(a): Low-power image of the colonic wall, with
a few endometrial glands and stroma embedded in the
muscular layer.
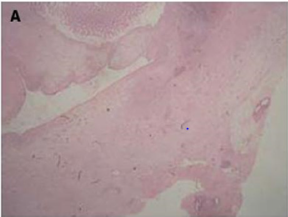
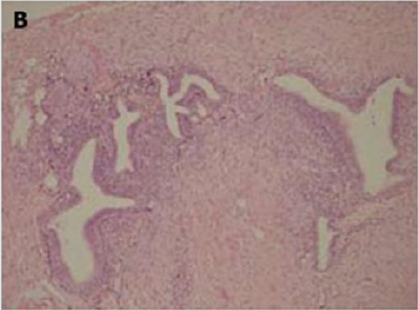
Figure 1(b): High-power view of the colonic wall, with
endometrial glands and stroma embedded in the smooth
muscle of the colon [12].
Endometriosis is considered a benign disorder; however, it
shares some of the characteristics of malignancy, such as abnormal
morphology, deregulated cell growth, cellular invasion, and
neoangiogenesis. The glandular epithelium occasionally displays
DNA aneuploidy. In vitro evidence suggests that endometriosis
may have a monoclonal origin. In addition to being monoclonal,
endometriotic deposits showed loss of heterozygosity in 28%
of lesions. In 2002, Nezhat et al. with immunohistochemistry,
found that alterations in bcl-2 and p53 may be associated with
the malignant transformation of endometriotic cysts [12]. The
development of a malignancy is a relatively common complication
of endometriosis. In fact, several publications have reported
malignant neoplasms arising from endometriosis. Most of
these publications are case reports or refer to a small series of
patients presenting either ovarian carcinomas with associated
endometriosis or invasive endometrioid adenocarcinomas
involving adjacent pelvic structures. Malignant transformation of
extraovarian endometriosis, including the intestinal tract, however,
has not been reported as frequently. The largest reported series
of neoplastic changes in gastrointestinal endometriosis includes
17 cases [10] (Figure 2(a-d)). Some studies suggest that the
development of malignancies may occur in up to 5.5 % of female
patients with endometriosis. Only 21.3% of the cases arise from
extragonadal pelvic sites, and endometriosis-associated intestinal
tumors are even rarer. Malignant transformation of primary
gastrointestinal endometriosis without pelvic involvement is
uncommon, and its real incidence is unknown. It can mimic a
primary gastrointestinal neoplasm. Most of these neoplasms
are carcinomas, but sarcomas and müllerian adenosarcomas have also been described. Petersen et al, in a large review of the
previously published endometrioid adenocarcinomas arising in
colorectal endometriosis, report less than 50 cases of neoplastic
transformation, 22 of which were adenocarcinomas. The others
included sarcomas and mixed müllerian tumors. The progression to
invasive cancer has been related with hyperestrogenism, either of
endogenous or of exogenous origin. A possible genetic background
favoring the onset of cancer has been reported in some patients
without hyperestrogenism and with a family history of cancer. The
anatomic distribution and frequency of these cancers parallel the
occurrence of which benign endometriosis is found at various sites.
In order to classify a malignancy as arising from endometriosis,
strict histopathologic criteria need to be fulfilled. Sampson first
proposed these criteria in the year 1925. He suggested that the
following should be fulfilled:
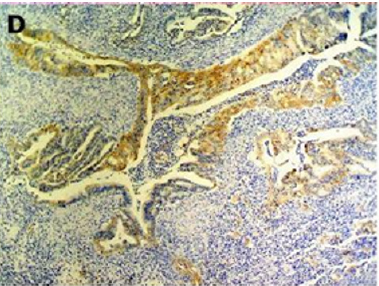
Figure 2(a): Rectal endometriod adenocarcinoma with
adjacent focus of endometriosis (hematoxylin-eosin, 20x).
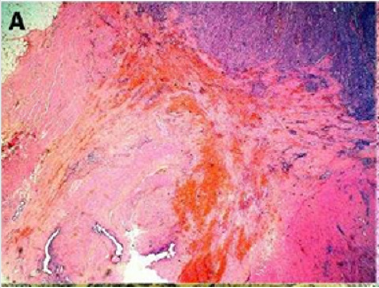
Figure 2(b): Rectal endometriod adenocarcinoma
endometriosis (hematoxylin-eosin, 100x).
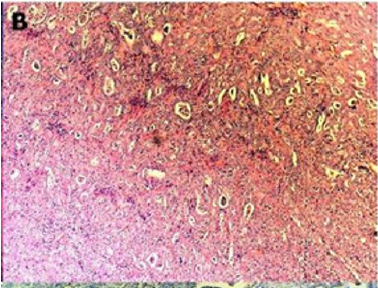
Figure 2(c): Cytokeratin 20 immunostaining negative
(100x).
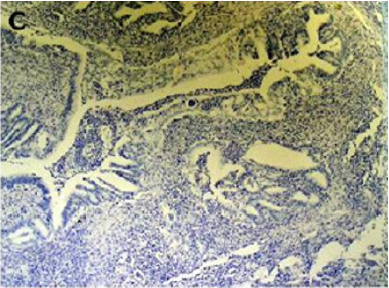
Figure 2(d): Cytokeratin 7 immunostaining positive (100x).
[10]
a) the presence of both malignant and benign endometrial
tissue in the same organ.
b) the demonstration of cancer arising in the tissue and not
invading it from elsewhere.
c) the finding of tissue resembling endometrial stroma
surrounding characteristic glands.
Years later, Scott suggested an additional qualification to
complete Sampson’s criteria: the demonstration of microscopic
benign endometriosis contiguous with the malignant tissue [10].
Endometriosis and its possible malignant changes should be taken
into account in the differential diagnosis of intestinal masses in
females. Also, clinical suspicion for malignancy should be aroused
in patients with abdominal pain or rectal bleeding and a previous
history of quiescent endometriosis. Recognition of these lesions
is important because of the different management required by
primary gastrointestinal neoplasms and by those arising from
endometriosis. These differences may have significant clinical
implications [10].
Endometriosis usually becomes apparent in the reproductive
years when the lesions are stimulated by ovarian hormones. Forty
percent of the patient’s present symptoms in a cyclic manner,
which are usually related with menses Pelvic pain, infertility and
dyspareunia are the characteristic symptoms of the disease, but the
clinical presentation is often non-specific [1]. Symptoms are initially
cyclical but may become permanent when the lesions progress. It is
difficult to establish a preoperative diagnosis of GI endometriosis,
because GI tract symptoms can mimic a wide spectrum of diseases,
including irritable bowel syndrome, infectious diseases, ischemic
enteritis/colitis, inflammatory bowel disease and neoplasm. GI
endometriosis patients present with relapsing bouts of abdominal
pain, abdominal distention, tenesmus [1], constipation and
diarrhoea. Rectal bleeding and pain during defecation may also
occur. Endometriosis infiltrating the muscularis propria may lead
to localized fibrosis in the bowel wall, strictures, and small or large
bowel obstruction. The true incidence of endometriosis causing
bowel obstruction is unknown, although complete obstruction of
the bowel lumen occurs in less than 1% of cases. Endometriosis
of the distal ileum is an infrequent cause of intestinal obstruction,
ranging from 7% to 23% of all cases with intestinal involvement.
The incidence of intestinal resection for bowel obstruction is 0.7%
among patients undergone surgical treatment for abdominopelvic
endometriosis [1]. Rectal bleeding may be caused by mucosal injury
during the passage of stools through a stenosed colon with the
intramural endometriotic tissue increased at the time of menses if
it occurs. Colonic mucosa heals rapidly, and no signs are detectable
at endoscopy [1] (Table 1).
Table 1:
Differential diagnosis [1]
a) irritable bowel syndrome,
b) infectious diseases,
c) ischemic enteritis/colitis,
d) inflammatory bowel disease
e) neoplasm
f) Other causes of intestinal obstruction (Acute/chronic,
small/large bowel)
A precise diagnosis about the presence, location and extent of
rectosigmoid endometriosis is required during the preoperative
workup because this information is necessary in the discussion
with both the colorectal surgeon and the patient. Furthermore,
almost all patients with intestinal endometriosis have lesions
in multiple pelvic locations and it is difficult to know what
symptoms are caused by the intestinal disease versus the pelvic
disease. In particular, in the case of sigmoid endometriosis, the
lesion cannot be suspected at clinical examination, which is why
sigmoid endometriosis is often diagnosed only during surgery.
Although several radiological techniques have been proposed for
the diagnosis of bowel endometriosis, data are inconclusive, and no
gold standard is currently available [13].
Colonoscopy
Although endoscopic diagnosis of colonic endometriosis has
been reported, the mucosa is usually normal or shows minimal
mucosal abnormalities, friability, extrinsic process or fibroses
stenoses [1]. Endoscopic biopsies usually yield insufficient tissue
for a definitive pathologic diagnosis as endometriosis involves
the deep layers of the bowel wall [14]. Endometriosis can induce
mucosal changes without any specific pattern, which mimic
findings of other diseases such as inflammatory bowel disease,
ischemic colitis or neoplasm [1]. Colonoscopy is helpful to rule out
colorectal malignancy [11].
Double Contrast Barium Enema
Radiologically, lesions of endometriosis are either of
constricting and polypoid type or both. On barium studies,
radiographic findings caused by implants in the ileum are similar to
those in the colon. Rectosigmoid or cecal endometriosis on double
contrast barium enema studies is seen as an extrinsic mass with
speculation and tethering of folds [1]. Shortening or flattening of
the bowel wall, crenulation of the mucosa, or a combination of
these factors [15], Double-contrast barium enema may be effective
in determining the precise location of the endometriotic nodules,
but it cannot clearly demonstrate the depth of parietal involvement.
Furthermore, the experience of the radiologist in the diagnosis of
bowel endometriosis remains a critical limit of this technique [13]
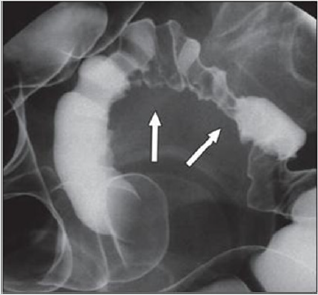
(Figures 3 & 4(a & b)).
Figure 3: Thirty-four years old woman with suspected intestinal implants of endometriosis. A and B, Lateral A and oblique B
spot images show three endometriotic lesions exhibiting extrinsic mass effect with crenulation of contour and speculation that
are direct signs of infiltration of bowel wall (arrows). Small polypoid lesion (arrowhead) is benign tubular adenoma confirmed
at surgery [15].

Figure 4(a): Twenty-eight years old woman with suspected intestinal implants of endometriosis and finding of rectal
localization of intestinal endometriosis. DCBE image shows extrinsic mass effect and speculation (arrow) of rectal wall that
appears infiltrated [15].
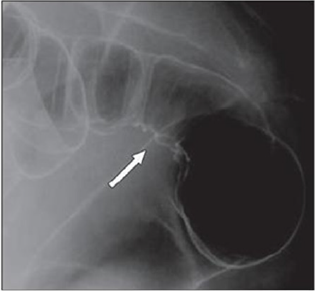
Figure 4(b): Twenty-three years old woman with suspected intestinal implants of endometriosis. DCBE examination showing
pathologic pelvic process involving bowel serosa at rectosigmoid junction. Finding of extrinsic mass effect and speculation
(arrows) owing to poor wall distention after air insufflation suggesting wall infiltration [15].
Transvaginal Us
Transvaginal ultrasonography can be useful not only in the
first-line exploration of the pelvic cavity, but also in diagnosing
rectosigmoid endometriosis. However, relevant limitations of
transvaginal ultrasonography consist in the impossibility of
determining the exact distance of rectal lesions from the anal margin
and of evaluating precisely the depth of rectal wall involvement. In
addition, locations above the rectosigmoid junction might be beyond
the field of view of a transvaginal approach and limited by the
presence of air for a transabdominal approach [15]. Transvaginal
us combines with rectal water contrast is more accurate than TVS
in diagnosing rectal infiltration reaching at least the muscularis
propria in women with rectovaginal endometriosis. However, this
exam cannot determine whether the infiltration reaches the rectal
submucosa. RWC-TVS may be more painful than TVS, therefore
it could be used when TVS cannot exclude the presence of rectal
infiltration in women with rectovaginal endometriosis [15] (Figure
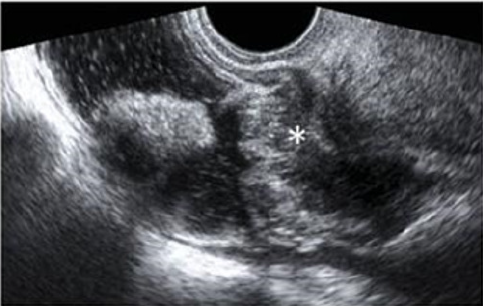
5).
Figure 5: A large rectovaginal nodule infiltrating the bowel muscularis (indicated by the asterisk) demonstrated by Rectal
Water Contrast- Transvaginal Sonography (RWC-TVS) [16].
CT & MSCT
CT is not the primary imaging modality for evaluation of
bowel endometriosis, although it can occasionally demonstrate
a stenosing rectosigmoid mass. Multislice CT (MSCT) has a great
potential for detecting alterations in the intestinal wall, especially
if it is combined with enteroclysis (MSCTe). Biscaldi et al carried
out a study on 98 women with symptoms suggestive of colorectal
endometriosis and MSCTe identified 94.8% of bowel endometriotic
nodules [1]. Biscaldi et al reported the usefulness of multislice CT
combined with distention of the colon by rectal enteroclysis for
bowel endometriosis. The sensitivity was 98.7% and specificity
was 100% in identifying women with intestinal endometriosis.
This method is thought to be very helpful for diagnosing intestinal
endometriosis, but requires bowel preparation, such as the need
for a low-residue diet for 3 d, drinking of 4-6 doses of a granular
powder dissolved in 500 mL of water per dose and intravenous
administration of iodinated contrast medium. This technique is
thus inappropriate for patients with obstructive symptoms or
allergy to iodinated contrast medium [3] (Figures 6 & 7).
Figure 6: Endometriotic nodule infiltration the muscular layer, A: Axial MSCTe image of the abdomen, the arrow indicates
the endometriotic nodule. The lesion is enhanced, and it infiltrates the bowel wall involving the muscular layer. B: Coronal
reconstruction demonstrating the extension of the sigmoid endometriotic nodule (indicated by the arrow) C: Formaldehydefixed
resected bowel segment, the endometriotic nodule of the sigmoid colon was previously demonstrated by MSCT [14].
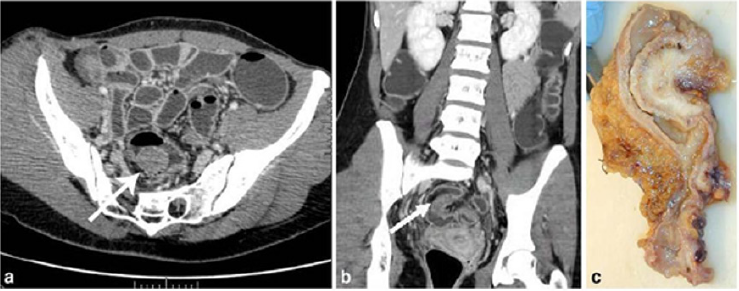
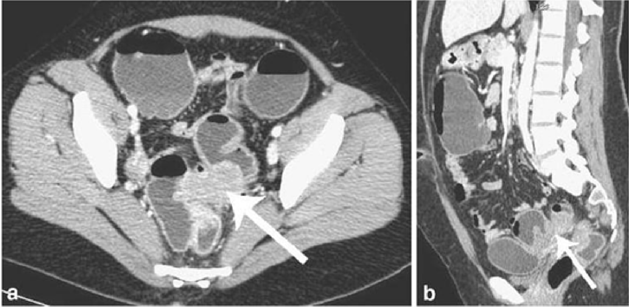
Figure 7: Endometriotic nodule infiltration the muscular layer, A: Axial MSCTe image of the abdomen, the arrow indicates
the endometriotic nodule. The lesion is enhanced, and it infiltrates the bowel wall involving the muscular layer. B: Coronal
reconstruction demonstrating the extension of the sigmoid endometriotic nodule (indicated by the arrow) C: Formaldehydefixed
resected bowel segment, the endometriotic nodule of the sigmoid colon was previously demonstrated by MSCT [14].
Magnetic Resonance Imaging (MRI)
Magnetic resonance imaging (MRI) has a high sensitivity (77%-
93%) in the diagnosis of bowel endometriosis. The depth of rectal
wall infiltration by endometriosis is poorly defined by MRI. A
combination of MRI and rectal endoscopic ultrasonography (EUS)
has recently been proposed. When retroperitoneal infiltration is
present, it is mandatory to know if the bowel wall is involved in
order to identify patients requiring bowel resection. Both rectal
EUS sensitivity and negative predictive value range from 92% to
100%. The specificity and positive predictive value are rather poor,
which are 66% and 64%, 83% and 94%, respectively, as reported
in two different studies [1]. Imaging examination is thus essential
for the preoperative diagnosis of intestinal endometriosis, but
some reports have described preoperative confusion between this
disease and cancer according to colonoscopy and CT with barium
enema, particularly in patients with lesions involving the mucosal
surface. In such patients, MRI is helpful for differential diagnosis.
In a typical endometrial lesion, MRI showed signal hyperintensity
on T1-weighted imaging and signal hypointensity on T2-weighted
imaging. However, smooth muscle components are reportedly
recognized frequently in endometrial lesions. In such lesions, as
seen in the present case, MRI indicates signal hypointensity on
both T1- and T2-weighted imaging, and differential diagnosis from
other diseases such as cancer and gastrointestinal stromal tumor
is thus difficult. In fact, Chapron et al reported that MRI specificity
for deeply infiltrating endometriosis was 97.9%, but sensitivity was
only 76.5% [3] (Figures 8 & 9).
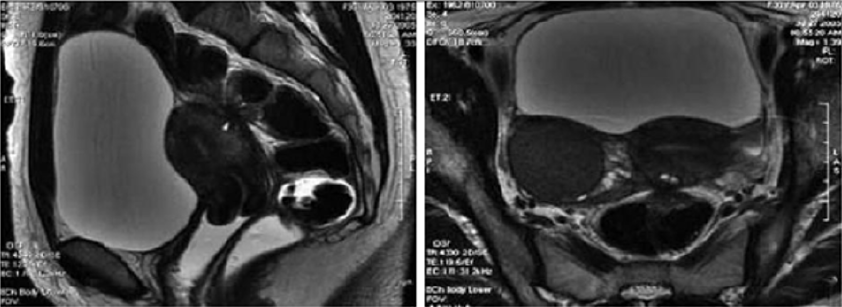
Figure 8: T2- weighted axial view: fecal matter attached to the rectal wall, simulating thickening of the rectal wall [17].
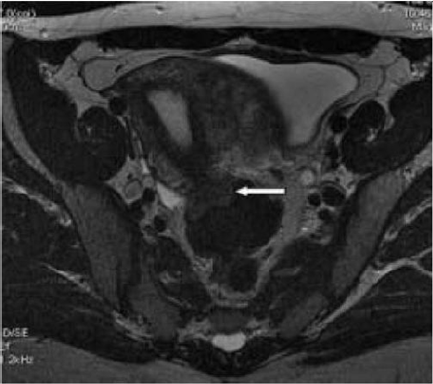
Figure 9: T2- weighted sagittal (a) and axial (b) views.
Nodule of the rectosigmoid junction adhering to the posterior surface of the uterus [17].
Transrectal EUS
The involvement of the colon is difficult to detect because the
implants rarely invade through the intestinal mucosa. For this
reason, the rectal ultrasound is of primary importance to assess the
rectal involvement [11]. Also, the depth of rectal wall infiltration
by endometriosis is poorly defined by MRI. A combination of
MRI and rectal endoscopic ultrasonography (EUS) has recently
been proposed. When retroperitoneal infiltration is present,
it is mandatory to know if the bowel wall is involved in order
to identify patients requiring bowel resection [1]. Endoscopic
ultrasonography is also a useful and noninvasive examination for
the diagnosis of intestinal endometriosis. Sensitivity and specificity
are reportedly about 97% for the diagnosis of rectal involvement in
patients with known pelvic endometriosis. In addition, EUS-FNAB
provides accurate tissue and may be the only procedure for correct
preoperative diagnosis of intestinal endometriosis, but the overall
specificity, sensitivity and accuracy of EUS-FNA for neoplasms
of the gastrointestinal tract are reportedly 88%, 89% and 89%,
respectively [3]. Among these examinations, it is considered that
MRI and EUS (and/or EUS-FNAB) are the most useful examinations
for intestinal endometriosis. However, it is important to perform
valuable examinations for diagnosis of intestinal endometriosis,
including radiological, histological and etiological examinations, as
the condition basically involves a benign lesion requiring minimally
invasive treatment [3] (Figure 10).
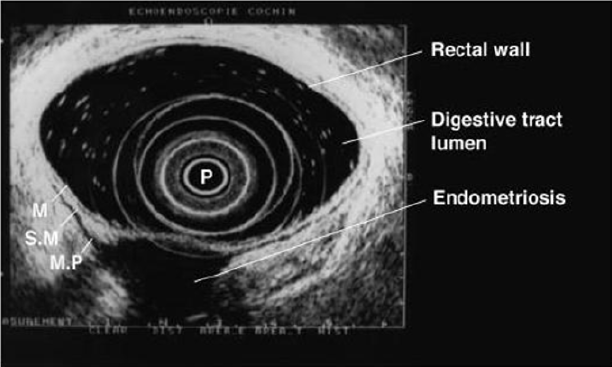
Figure 10: Rectal endoscopic ultrasonography showing a uterosacral endometriosis nodule (2 cm x 3 cm) with bowel infiltration.
P = probe, M = mucosa, SM = submucosa, MP = muscularis propria [18].
7- Serum Markers
There is a great interest in the use of serum markers to diagnose
endometriosis, but they are not sufficiently accurate for use in
clinical practice. Cancer antigen CA-125 has been used to monitor
the progress of endometriosis [16]. CA19-9 has a lower sensitivity
than CA-125, and cytokine interleukin-6 may be more sensitive and
specific than CA-125 [1]. Mol et al reported a systematic review of
the diagnosis of endometriosis and concluded that serum CA125
level may be elevated in endometriosis, but this measurement had
no value as a diagnostic tool compared to laparoscopy [3].
Laparoscopy
Laparoscopy is a primary diagnostic and therapeutic tool
providing the opportunity to explore the abdominal cavity and
obtain biopsies. The magnified vision enables the surgeon to operate
with the best possible exposure. Although it was once believed that
intestinal endometriosis was best managed by hormonal regimens
or surgical castration, the advent of laparoscopic surgery has
dramatically changed this approach [11] (Table 2).
Table 2:
Despite being a gynecologic pathology, deep infiltrating
endometriosis is not of exclusive gynecologic concern. A
multidisciplinary approach involving urologists and colorectal
surgeons therefore is recommended strongly for complete
evaluation and correct management. A minimally invasive approach
offers convenient advantages concerning the surgical management
of multifocal deep infiltrating endometriosis. Traditionally, radical
surgery [17] was considered the best measure to prevent disease
relapse. However, because of the prevalence of endometriosis
among women of reproductive age and the advances in surgical
techniques, minimally invasive conservative surgery now is
encouraged more [18]. Treatment must be individualized, taking
the clinical problem in its entirety into account, including the impact
of the disease and the effect of its treatment on quality of life. Pain
symptoms may persist despite seemingly adequate medical and/or
surgical treatment of the disease. In such circumstances, a multidisciplinary
approach involving a pain clinic and counselling should
be considered early in the treatment plan. It is also important to
involve the woman in all decisions; to be flexible in diagnostic
and therapeutic thinking; to maintain a good relationship with
the woman, and to seek advice where appropriate from more
experienced colleagues or refer the woman to a centre with the
necessary expertise to offer all available treatments in a multidisciplinary
context, including advanced laparoscopic surgery
and laparotomy [19]. The objective of the treatment in pelvic
endometriosis is to cease the endometrial stimulus in order to
ameliorate the symptoms. Thus, danozol, gonadotropin- releasing
hormones, oral contraceptives, and prostaglandin inhibitors can be
used. The conclusive treatment of endometriosis is total abdominal
hysterectomy, bilateral salpingo-oophorectomy and removal of
all endometrial foci. Because malignant transformation cannot be
excluded preoperatively and medical treatment may cause fibrosis,
the definitive treatment is surgical. Also, in the case of intestinal
obstruction and severe rectal and abdominal pain, surgery is
indicated. The main objective of surgery is the resection of the
affected bowel segment, enabling the histopathological examination
of the resection material. Limited surgery, such as excision or
cauterization of superficial lesions, following confirmation through
frozen section analysis could be performed. In conclusion, intestinal
endometriosis is a disease that may imitate various gastrointestinal
system diseases. The definite diagnosis could only be done by
histopathologic confirmation, since there are no pathognomonic
radiological or colonoscopic findings. In female patients who have
unexplained digestive complaints, endometriosis should also be
considered in the differential diagnosis [20].
It depends on the patient’s age and intention to conceive. Bowel
resection is indicated if there are symptoms of obstruction or
bleeding, and if malignancy cannot be excluded. In patients of childbearing
age, resection of the involved colon followed by hormonal
treatment may be sufficient; otherwise, hysterectomy and bilateral
oophorectomy is the treatment of choice [21]. Medical suppressive
therapy may be beneficial in some patients with symptomatic
rectovaginal endometriosis, but often it is either ineffective or
only temporarily effective, whereas surgical therapy is effective in
relieving pain conditions. Other studies have shown that operative
therapy of rectovaginal endometriosis does not modify reproductive
prognosis but significantly reduces pain and improves quality
of life. The best long-term results are obtained after complete
excision of the endometriotic tissue [22]. The surgeon’s judgment
on bowel involvement with the consequence of bowel resection is
of the utmost importance [22]. Redwing has suggested a severity
scoring system for intestinal endometriosis based on the form of
surgical management required: grade I, superficial seromuscular;
grade II, partial thickness to mucosa; grade III, full thickness;
grade IV, segmental. The surgical approaches to intestinal disease
include simple excision (with cautery or laser), mucosal skinning,
full thickness disc excision with primary closure, and formal bowel
resection [23]. Full thickness disc resection of bowel endometriotic
lesion is often incomplete, at least one-third of patients with
bowel endometriosis treated by full thickness disc resection have
persistent disease. The surgeons must always weigh the risk
of potential complications of surgery against the benefit of the
complete removal of bowel endometriotic lesions. To date, no clear
guideline exists for the pre-operative assessment of patients with
suspected endometriosis; therefore, bowel resections should only
be performed after a careful pre-operative evaluation of patients’
symptoms and a radiological examination of the bowel [23]. Bowel
resection can be performed according to previously published
criteria (Remorgida et al.): single lesion >3 cm in diameter, single
lesion infiltrating >50% of the bowel wall, three or more lesions
infiltrating the muscular layer [15].
The collaboration of a laparoscopically skilled gynaecologist
and colorectal surgeon has been recognised as ideal in the surgical
management of colorectal endometriosis. Patients undergo bowel
preparation 24 h prior to surgery with a Fleet® ACCU-PREP®
Bowel Cleansing System (C.B. Fleet Co., Braeside, Vic., Australia)
[24]. Prophylactic anticoagulant therapy was given the evening
before the operation, and prophylactic antibiotic therapy was
given at the beginning of the operation [25]. Surgery is performed
with the patient in the lithotomy position. Five ports are used with
placement as shown in Figure 11. Five-millimetre 0° and 5-mm
30° endoscopes are used and most dissection is undertaken using
the harmonic scalpel (Ethicon Endo-Surgery, Inc., Cincinnati, OH,
USA). Pneumoperitoneum is maintained at 12-14 mmHg with a
flow rate of 40 L/min. For an anterior segmental bowel resection,
the descending colon is mobilised up to the level of the splenic
flexure. The ureter is identified crossing the pelvic brim on the
left, and mobilisation continued inferiorly to open the para-rectal
space medial to the uterosacral ligament. The right mesocolon
is then opened and dissection extended down to the right pararectal
space medial to the uterosacral ligament. The right ureter
is identified during this dissection. The sigmoid colon is then
elevated with bowel grasping forceps, and the space posterior
to the sigmoid mesocolon is opened. During this dissection the
inferior mesenteric vessels are identified and divided using an
endoscopic linear cutter 45 mm (ATW45 Ethicon Endo-Surgery,
Inc.) once the requirement for a bowel resection is established.
Further elevation of the sigmoid colon allows the posterior
dissection to continue inferiorly to the presacral plane, allowing
mobilization of the rectum. Having mobilized the rectosigmoid
laterally and posteriorly, the rectum is then dissected free from
the posterior cervix. This is the most difficult part of the dissection
and an attempt is made to free disease from the posterior cervix
and posterior vaginal wall as completely as possible. If there is coexisting
invasive uterosacral disease, this is excised end bloc with
the affected rectal segment. The inferior dissection is complete
when the normal tissue in the rectovaginal septum is encountered.
Once the level of rectal transection is identified, the mesorectum
is divided at that point leaving the rectal tube. An endoscopic
articulating linear cutter 45 mm (ATG45 Ethicon Endo-Surgery,
Inc.) is introduced and applied transversely across unaffected
distal rectum. The stapler is fired to separate the affected rectal
segment from the distal rectal stump. Two firings may be required.
The lower right 12 mm port site is then converted to a minilaparotomy
incision, approximately 3-4 cm in length. The affected
rectal segment is then delivered through this wound, clamped and
divided above the level of disease. The anvil from an endoscopic
curved intraluminal stapler 29 mm (ECS29 Ethicon Endo-Surgery,
Inc.) is secured into the proximal colon with a purse-string suture.
The proximal segment is then returned to the abdominal cavity
and the mini laparotomy wound closed. Pneumoperitoneum is reestablished.
The ECS29 is passed transanally, and the distal rectal
stump is elevated. The circular stapling device is opened, passing
a metal spike through the distal rectal stump adjacent to the staple
line. The anvil within the proximal segment is then docked to the
spike and the circular stapling device closed. The circular stapling
device is then fired to complete the anastomosis. After removing
the transanal stapler, an integrity check is performed by distending
the rectum with Betadine after occluding the sigmoid colon at the
pelvic brim. Further check of integrity is undertaken by instilling
air into the rectum after flooding the pelvis with saline. A 17-gauge
drain is left in the operative site, after which all ports are removed
[24]. Terminal-to-terminal anastomoses were classified according
to distance from the anus as high/medium (>8 cm), low (5-8 cm)
or ultralow (<5 cm). The choice to perform primary ileostomy
or colostomy was based on intraoperative findings [26]. For a
disc excision, a lesser degree of descending colon mobilization is
required, less posterior rectosigmoid dissection may be required
and there is no requirement to divide the inferior mesenteric vessels.
The principles of pelvic dissection are otherwise as described. Once
the rectal disease has been identified and isolated, a figure of eight
suture is placed through the lesion. An ECS33 stapling device is
passed transanally with the anvil intact. The device is then opened
and angled towards the rectosigmoid lesion. The suture is grasped
with laparoscopic forceps, and the disease drawn down into the
open stapler. The stapling device is then closed, rotated slightly to
ensure that the posterior rectal wall has not become entrapped,
and then fired. An anterior arc of rectal wall containing the lesion
is therefore removed and the rectal wall stapled in a single action.
Rectosigmoid integrity checks are then undertaken as described
above [24]. The extent of the lesions as well as the severity
of the symptoms justifies the extensive nature of the surgery
undertaken. The findings of additional areas of the bowel that are
macroscopically normal but microscopically involved, as well as
involvement of the lymph nodes, suggest to us that simple local
excision of a disc may on occasions be inadequate to remove the
whole of the involved area of the bowel [12]. There are no data to
justify hormonal treatment prior to surgery to improve the success
of surgery [19]. However, according to ESHRE guidelines Postoperative
treatment for endometriosis in general might include
danazol or a GnRH agonist for 6 months after surgery as it reduces
endometriosis associated pain and delays recurrence at 12 and
24 months compared with placebo and expectant management.
However, postoperative treatment with a COC is not effective [19].
Figure 11: Placement of five port sites used in Surgery.
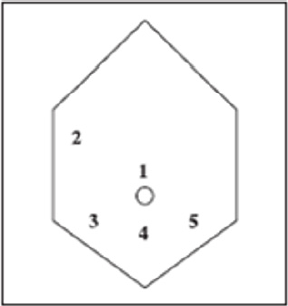
The risk of complications depends on the clinical conditions,
such as the level of bowel stenosis, opening of the vaginal wall, the
extent of endometriosis infiltration, and the surgeon’s experience.
Moreover, the possibility of performing this kind of surgery
(complete eradication with colorectal surgery) in a referral center
reduces the risk of complications and improves clinical outcomes.
Indeed, women who undergo intestinal surgery are at higher risk
of complications mainly in the short-term, but close surveillance
reduces the risk of need for reintervention and allows a good
recovery within a few weeks [27]. Complications include:
a) Internal hemorrhage
b) Bowel fistula
c) Vaginal fistula
d) Retention of urine
e) Constipation
f) Abdominal wall hematoma
g) Ureteral injury and stenosis
h) Bladder perforation
i) Uterine perforation
j) Cystitis
k) Adynamic ileus
l) Mechanical bowel obstruction
m) Peritonitis
n) Peritoneal effusion
The indications of colorectal resection for endometriosis are
controversial, and the likely risk/benefit ratio must be discussed
with each patient. No menstrual pelvic pain, pain on bowel
movement, cramping, and cyclic rectal bleeding improved or
disappeared in all the women concerned, in keeping with previous
studies of colorectal resection for endometriosis. dysmenorrhea,
dyspareunia, pain on defecation, and no menstrual pelvic pain
improved significantly, on the basis of visual analog scores, whereas
no impact was noted on pain on bowel movement, lower back
pain, or asthenia. Recent results confirm those of Redwing and
Wright, showing that women with dysmenorrhea, dyspareunia,
pain on defecation, or no menstrual pelvic pain associated with
complete endometriotic obliteration of the sac of Douglas are the
best candidates for extensive resection [28]. Bowel resection is not
completely free of recurrence of endometriosis, but the incidence
of recurrence is significantly lower [29]. In conclusion, laparoscopic
rectosigmoid resection and end-to-end anastomosis seem safe and
effective in women with deep infiltrating colorectal endometriosis,
where the bowel lumen is largely restricted, and bowel function
is greatly impaired. Results of long-term follow up demonstrate
significant reductions in painful and dysfunctional symptoms
associated with deep bowel involvement [30]. Laparoscopic
segmental colorectal resection for endometriosis is associated with
a significant improvement in quality of life and gynecological and
digestive symptoms [25].
Read More Lupine Publishers
Journal of
Surgery Articles :
https://surgery-casestudies-lupine-publishers.blogspot.com/
Read More
Lupine Publishers
blogger Articles:
https://lupinepublishers.blogspot.com



















